Advantages
Clinical and Industrial leads.
Starting from the statement that the paralysis due to spinal cord lesions is currently untreatable, the first potential and great advantage will be the spinal regeneration and the motor recovery.
It’s about a second therapeutic use, therefore it is well-known toxicity, doses and therapeutic- and side-effects. The use in humans is approved (FDA, AIFA) for different neurological, muscular and dermatological pathologies; as well as BoNT/A is utilized off label for several clinical uses which employ commercial formulas (BOTOX® Dysport®, Vistabex®, Xeomin®).
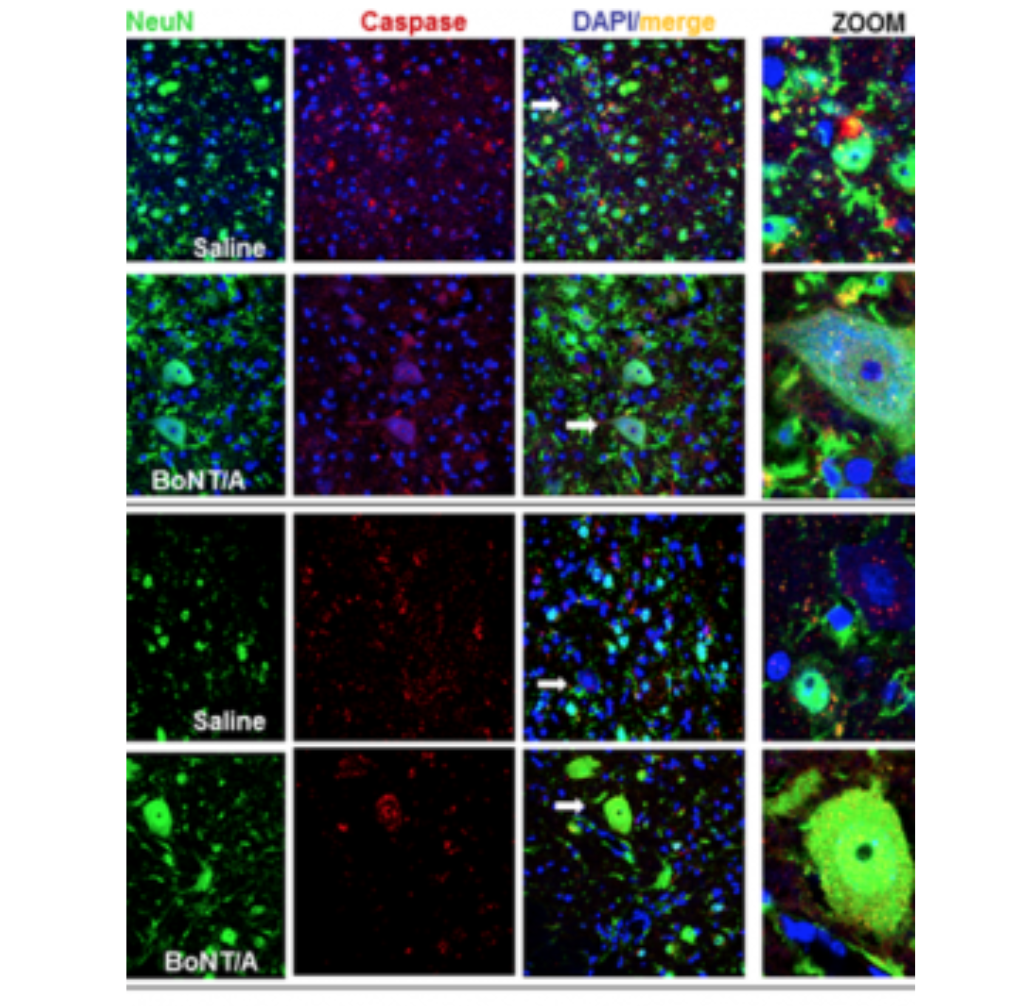
The animal testing (preclinical results) for the new therapeutic use is already carried out and the effectiveness demonstration, in spinally contused mice with complete hindlimbs paralysis, published (Vacca et al. Toxins, 2020). The utilized doses (15 picograms/mouse), with therapeutic effect, haven’t toxicity, are safe and lower than those used in clinical therapy (100U Botox ῀ 4,8 nanograms/human).
Thanks to its biological action a single treatment (a single dose administration) is able to have a therapeutic activity from 2 to 6 months, in consistent manner, avoiding countinuing and repeated administrations.