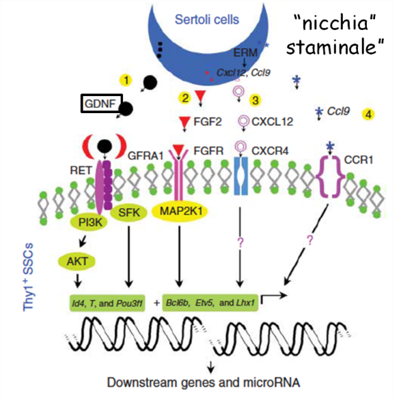
The efficacy of eicosapentaenoic acid (EPA) and (all omega-3s) – 4,7,10,13,16,19 docosahexaenoic acid (DHA), has been demonstrated in the treatment and prevention of male infertility of post-pubertal patients affected by alterations in sperm motility (asthenozoospermia) and morphology (teratozoospermia). In particular, the compositions of fatty acids have been found to be potentially useful exclusively in the treatment of subjects who have already developed spermatozoa, and not in pre-pubertal subjects in which sperm production has not yet occurred.
We propose a pharmaceutical composition, in particular polyunsaturated fatty acids of the ω-3 series, for the prevention and treatment of male infertility, with special focus on infertility due to azoospermia, caused to chemo/radiotherapy treatments in pre-pubertal subjects, in which cryopreservation cannot be done.