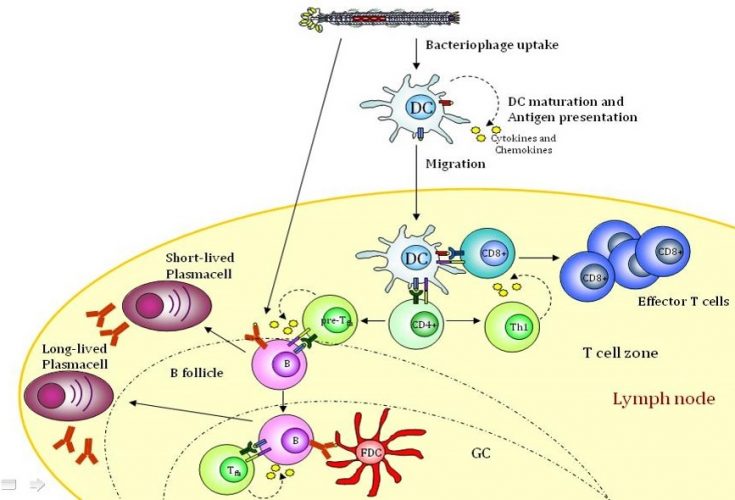
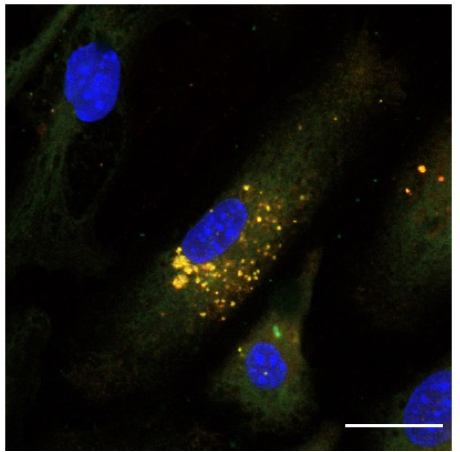
The invention relates to pharmaceutical compositions comprising lipid- bacteriophage conjugates, wherein lipid, as the glycolipid alpha-Galactosylceramide is immunologically active and the bacteriophage is engineered to bind to target cells and carry antigenic molecules capable of stimulating an immune response. Such bacteriophage is used to stimulate an immune response against cancer and against infectious agents.