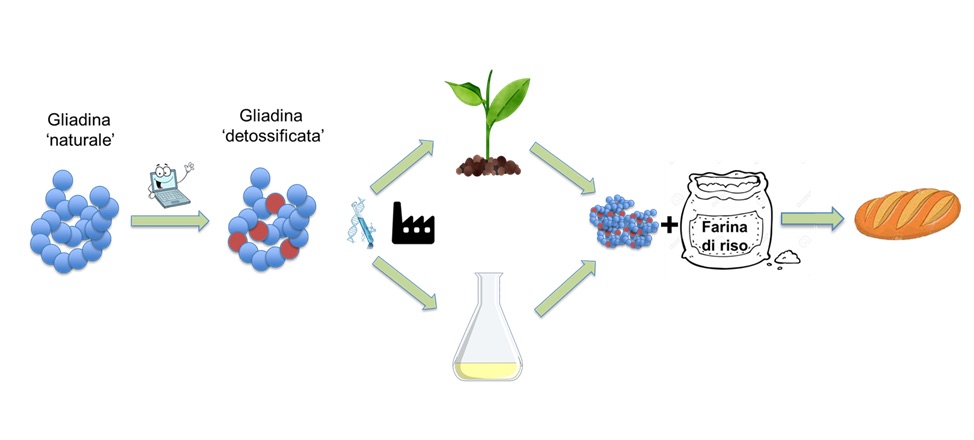
Elimination of gluten from the diet is to date the only effective therapy to achieve remission of symptoms and prevention of complications. The quality of life of coeliac patients is lower than that of healthy individuals because it is difficult to adopt a well-balanced and strict gluten-free diet. Gluten proteins contribute to the viscosity and generate the elasticity and toughness of doughs. In pasta, for example, the gluten lattice slows down the absorption of water during cooking by the starch, giving the product tenacity and elasticity; in bread, on the other hand, it retains the gas bubbles produced by the yeast, as well as giving cohesion, homogeneity, visco-elasticity and tenacity to the dough, and allows for a soft and elastic product that is pleasing to the palate. However, the numerous and very diverse gluten proteins are encoded by multiple genes at complex loci and this genetic complexity makes it impossible to generate gluten-free wheat or other gluten-containing cereals using classical genetic techniques (such as crossing).In gluten-free cereal-based foods, the gluten mesh is therefore replaced with gums and/or thickening substances, emulsifiers, and although considerable progress has been made to improve the palatability of gluten-free foods, often the industrial products available on the market are high in calories, have a lower nutritional value and are particularly expensive. Other proposed solutions to deal with ‘coeliac disease’ have focused on the development of chemical-physical treatments to break down the gluten present in food, drug development and the development of low-gluten wheat varieties, but have so far failed to identify an alternative diet.