For primary open angle glaucoma (POAG), most of the treatments currently focus on reducing aqueous humor formation, enhancing uveoscleral or conventional outflow, by lowering intraocular pressure (IOP) through medical or surgical means. However, limiting IOP levels only does not arrest of the progression of the glaucomatous damage (due to the fact that glaucomatous pathology are not exclusively due to an increase in IOP) and do not prevent patients from progressive vision loss. Therefore, other strategies are needed to reduce or reverse the progressive neurodegeneration.
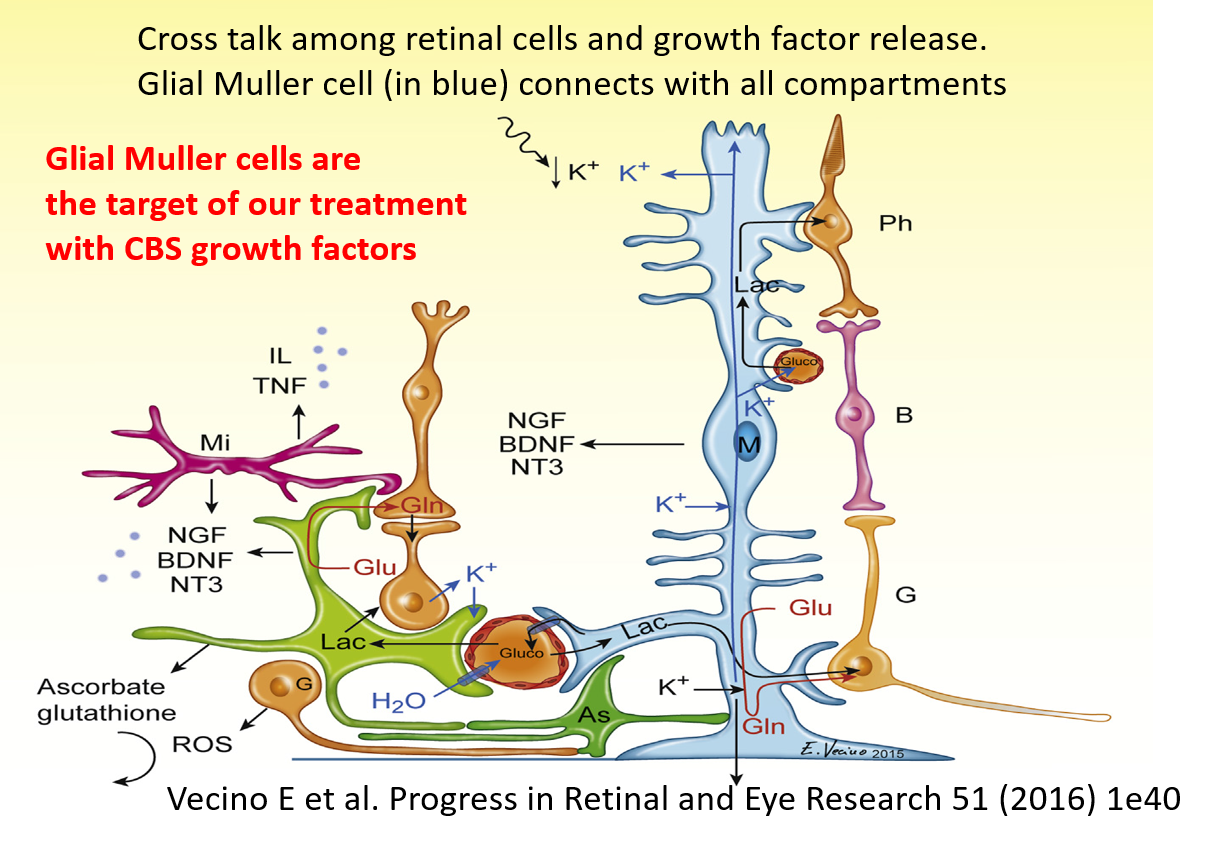
Recent pathophysiological acquisitions have directed international scientific research to explore and study molecules capable of exerting a potential neuroprotective or neuro- empowering effect on retinal ganglion cells, the optic nerve and the central optic pathways, with a direct mechanism of action on the nervous structures and therefore not necessarily linked to the hypotonizing effect. Currently at the level of neuroprotection there are several molecules being tested. Some examples: substances with antioxidant, anti-apoptotic action, and substances able to reduce glutamate excitotoxicity
However, in current clinical practice neuroprotection is currently offered to patients in combination with IOP treatment only according to clinician’s judgment and mostly through administration of nutraceuticals, but significant evidence on their validity and efficacy is missing.
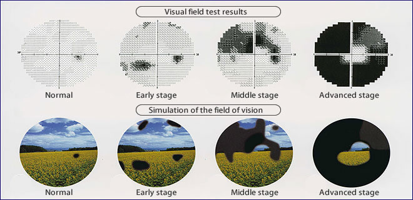
An effective and clinically validated neuro protective treatment accompanying IOP treatment and intervening at very early glaucoma stage, especially on young patients or patients with glaucoma familiarity would substantially change the impact of the disease.