Cancer is a complex pathology that can be caused by many factors (e.g. genetic predisposition, lifestyle, environmental factors, and viral infections) and about 100 different kind of tumours are known, belonging to six different main categories (carcinoma, sarcoma, myeloma, leukaemia, lymphoma, mixed type). More than 2.7 million new cases of cancer are diagnosed in Europe per year with one in four deaths that can be attributed to cancer. Thanks to the early diagnosis and increasingly personalised medicines, the 51% and 38% of the diagnosed tumours in women and men, respectively, are successfully treated, but unfortunately, the other still remain incurable. This is mainly because of the late diagnosis, lack of adequate therapies, aggressiveness and low compliance of some treatments which are responsible for heavy side effects.
In parallel, more than 5 million people are affected by chronic inflammatory diseases (e.g. rheumatoid arthritis, lupus, atopic dermatitis, and Crohn’s disease) progressively debilitating, undermining people’s quality of life and creating socio-economic burdens. Chronic inflammation can also be precursor of certain cancers. Broad immunosoppressive treatments are usually employed, but alternative/personalized approaches are highly required.
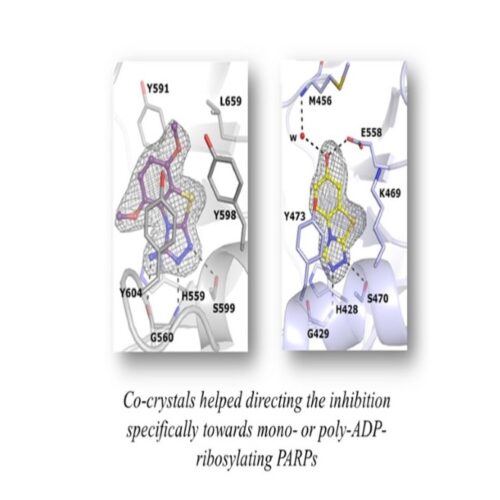
The lead compounds object of the patent inhibit selectively certain PARPs which have recently emerged as important players in cancer and inflammation, thus suggestion them as alternative therapeutic options.