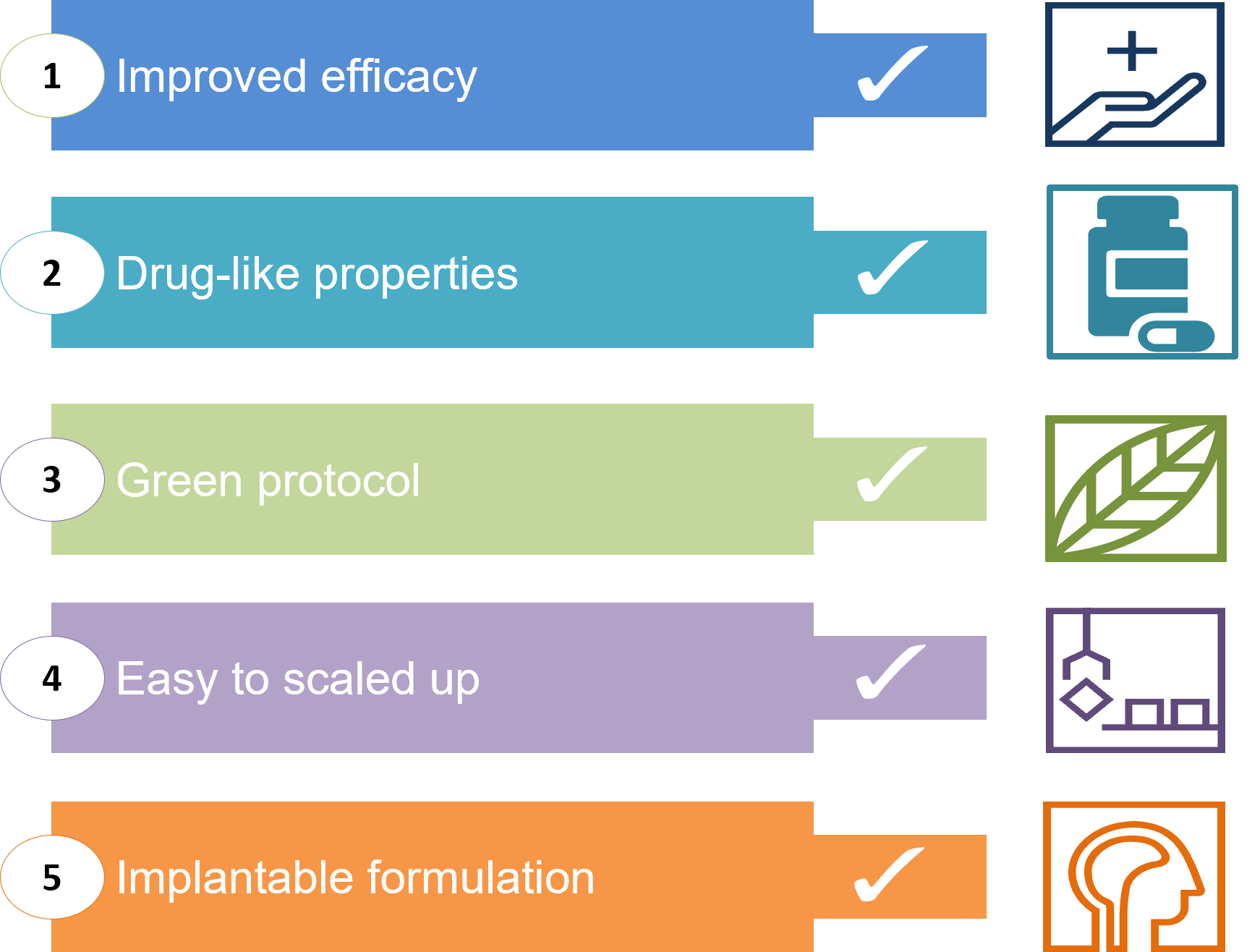
The present invention discloses small molecules 70-folds more effective in vitro than temozolomide on patient-derived GB tumoral cell lines, and with efficacy comparable to that of bevacizumab, but with improved drug-like characteristics. Compounds identified so far are at the “hit stage”. The inventors’ experience in hit identification utilizing all modern techniques, including both the chemistry and the biology competences led to the identification and refinement of high-quality hit series.