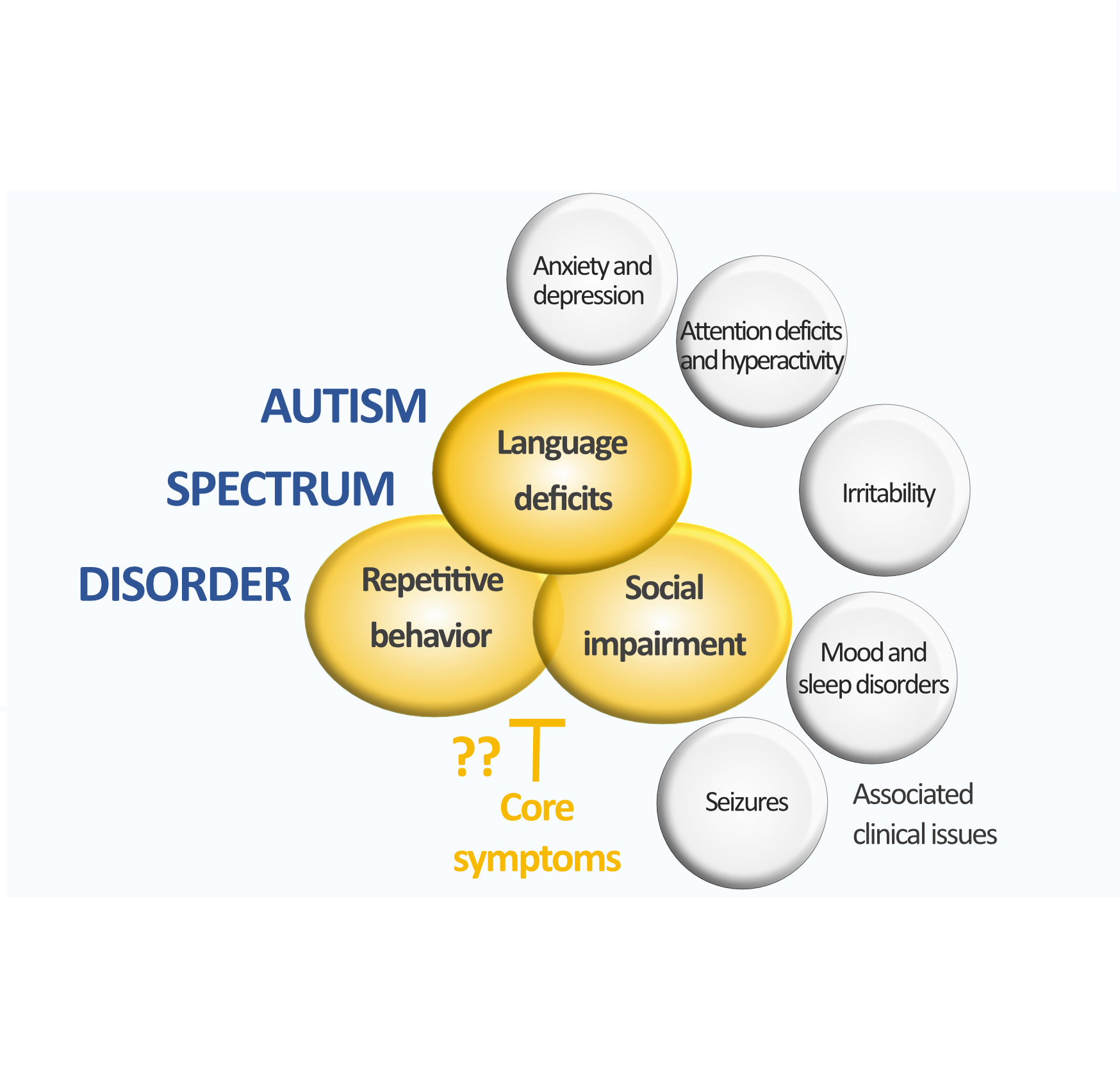
Autism Spectrum Disorder (ASD) refers to a highly prevalent group of neurodevelopmental disorders showing convergent cognitive/behavioral symptoms, including impairments in language, social communication and stereotypies, often coupled to anxiety and often accompanied by intellectual disability. Thousands different causative genetic lesions have been identified for ASD, mostly represented by de novo mutations or copy number variations splitting ASD de facto into an aggregate of rare genetic diseases with phenotypic convergence.
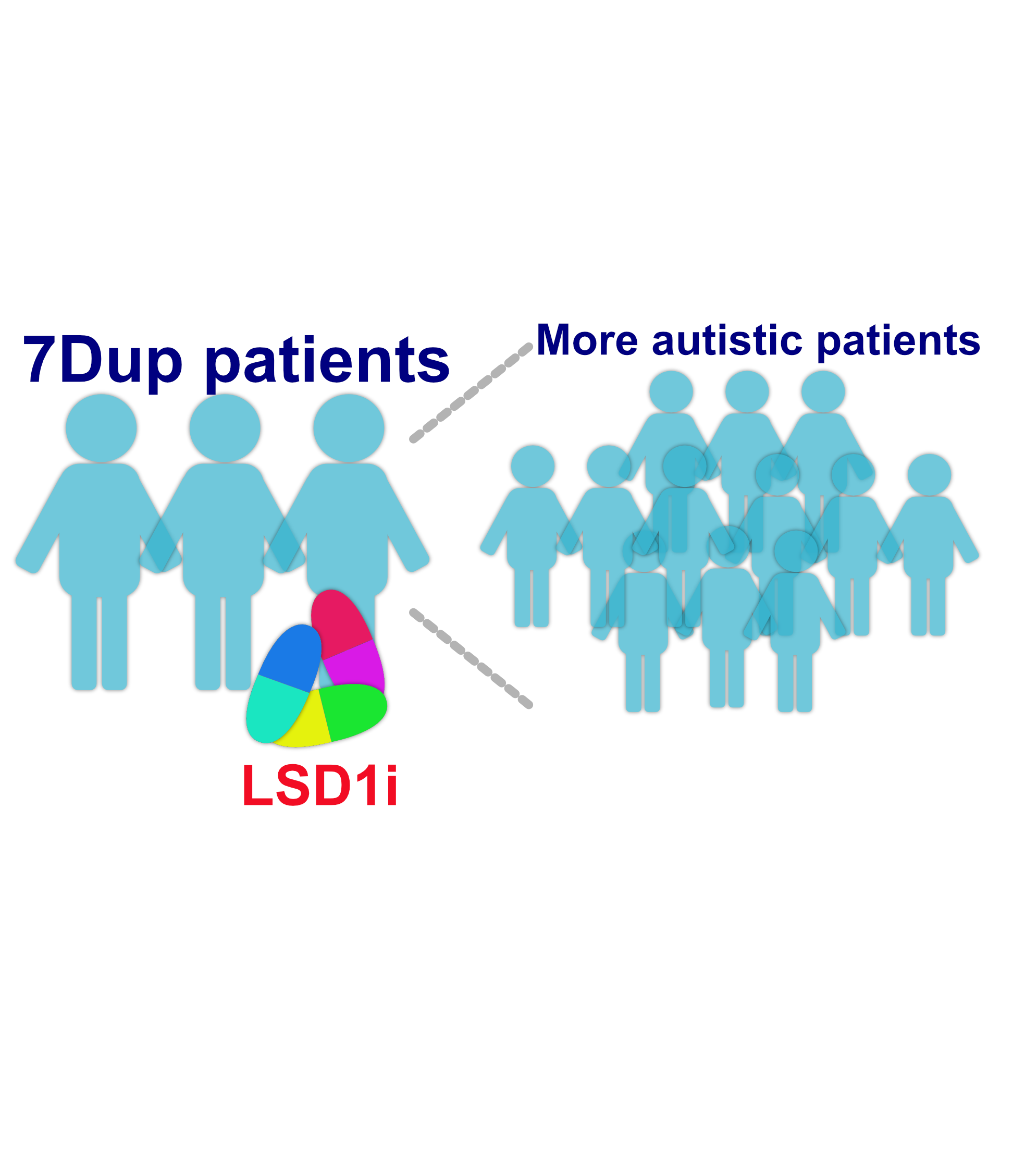
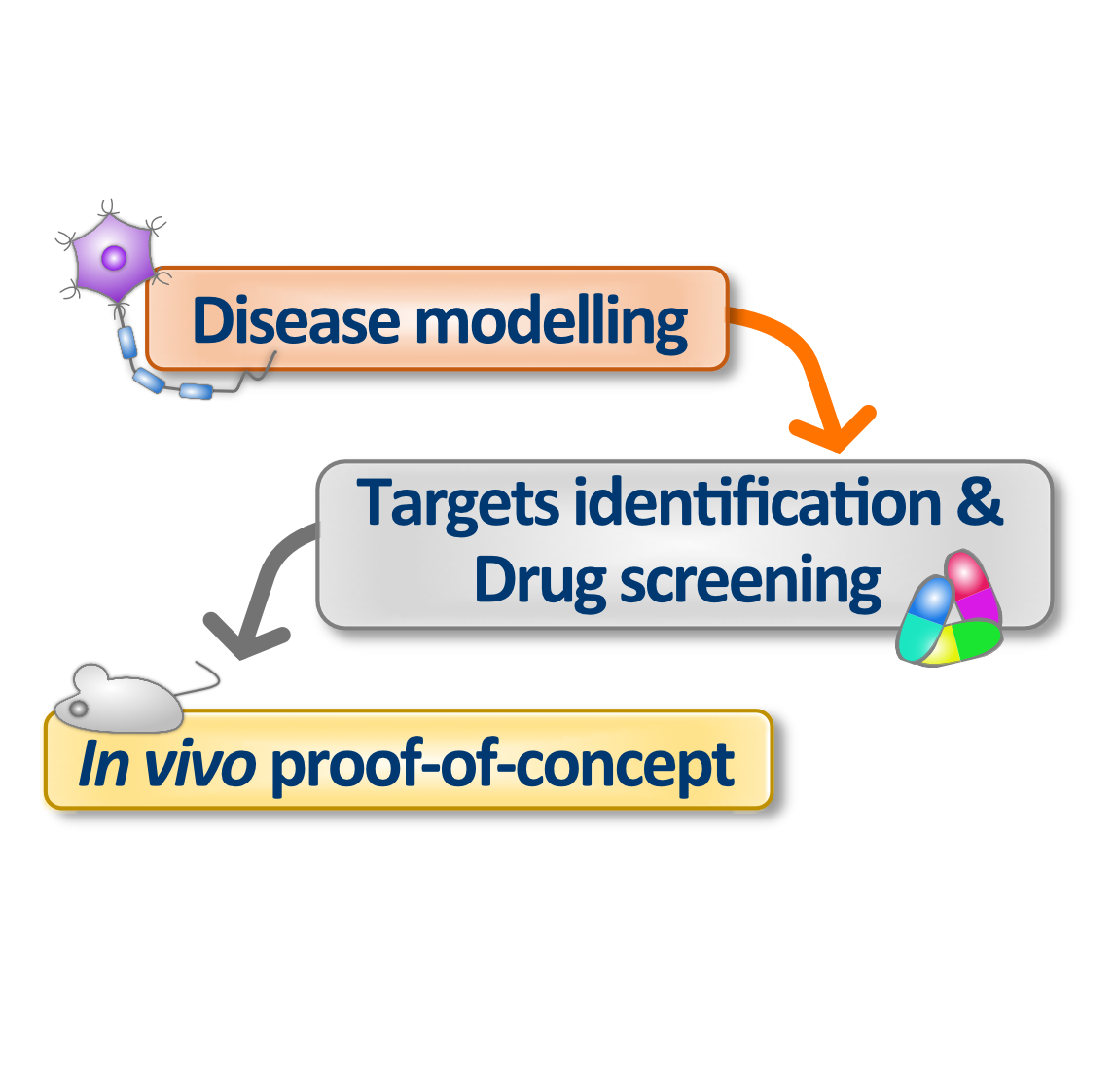
Prof. Giuseppe Testa’s research program aims at identifying molecular mechanisms and potential therapeutic interventions for certain rare subtypes of ASD, in particular the 7Dup-Autism Spectrum Disorder (7DupASD), where the abnormal duplication of the chromosomal region 7q11.23 has been recognized as the cause. Individuals affected by 7DupASD show language impairment and hyposociality along with other characterizing aspects of ASD. The team is investigating classes of epigenetic modulators to treat ASD by tackling key mechanistic dysregulations that underlie its core symptoms.