Roadmap
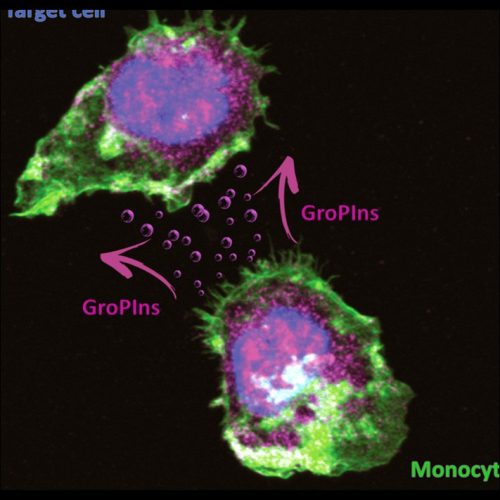
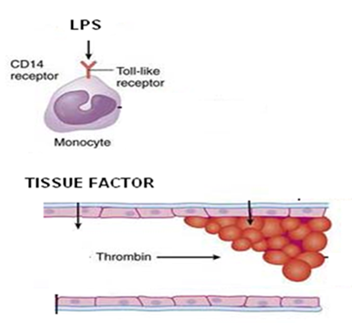
The team of the main inventor has developed virtually all the knowledge today available on this class of compounds, including its metabolism, catabolism, distribution, biological functions, also with reference to the differently phosphorylated glycerophosphoinositols.
This knowledge has to be complemented with expertises in developing preclinical and clinical studies on the proposed application, as described before.
At the same time, collaborations with a pharma ready to invest in this topic and with synthesis expertise could help in obtaining pure compound for proceeding with the studies in humans.
We believe that ideal partners are colleagues interested in the development of a non-steroidal antiinflammatory compound for which the efficacy of the topical application is already demonstrated (and the compound is commercialized for that), but it is in the need of the step forward, to exploit all its possible applications, starting from the septic shock described in this patent, and moving then to other applications that we already have in our pipeline (preliminary data).
We believe that this will be a winning partnership, beneficial for all, patients included (and most important).