Ulcerative colitis and Crohn’s disease are inflammatory bowel diseases that affect about 1% of the world’s population, especially young adults and children. These diseases are severe, chronic and often lead to complications such as cancer. Symptoms are often alike, are common to other benign diseases that affect about 30% of the population. This causes a delay in diagnosis in many cases: the tests that are available today aren’t specific for the disease and require lengthy processing.
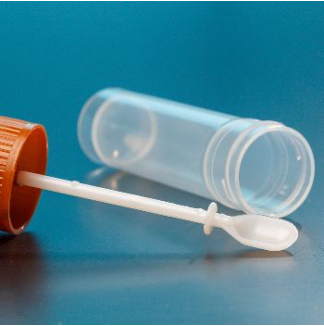
My team at the University of Padua has developed a new lab test so that patients get the right treatment, and no precious time and money are wasted. All it takes is a stool sample. Any lab equipped with a MALDI TOF analyzer can quickly and automatically analyze a large number of samples in a short time. Thanks to the innovative algorithm that can identify the specific profile for each disease, doctors can now rely on a fast, precise result, and patients can forget painful, invasive tests.
The number of people in Industrialized countries that are affected by IBDs is on the rise: (diagnostics will play a crucial role in rationalizing the burden of therapeutic costs on our health care systems).
We’re seeking a leading international medical diagnostic company to bring this tool to hospitals and labs worldwide: our successful prospective phase has proven this technology to be ready for certification. This diagnostic method is covered by an international patent application.