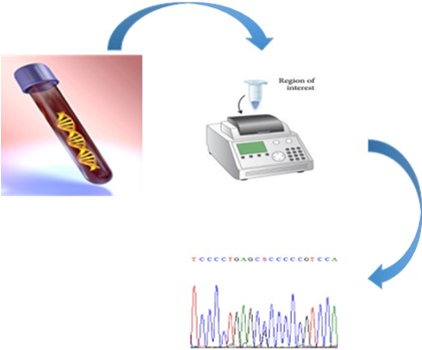
The present invention relates to a new diagnostic marker for Paget’s disease of bone (PDB) and bone tumors associated therewith, such as Giant Cell Tumor (GCT). This marker is represented by the ZNF687 gene that, when mutated, is responsible for an aggressive form of PDB. If not treated, these PDB patients usually undergo the neoplastic degeneration.