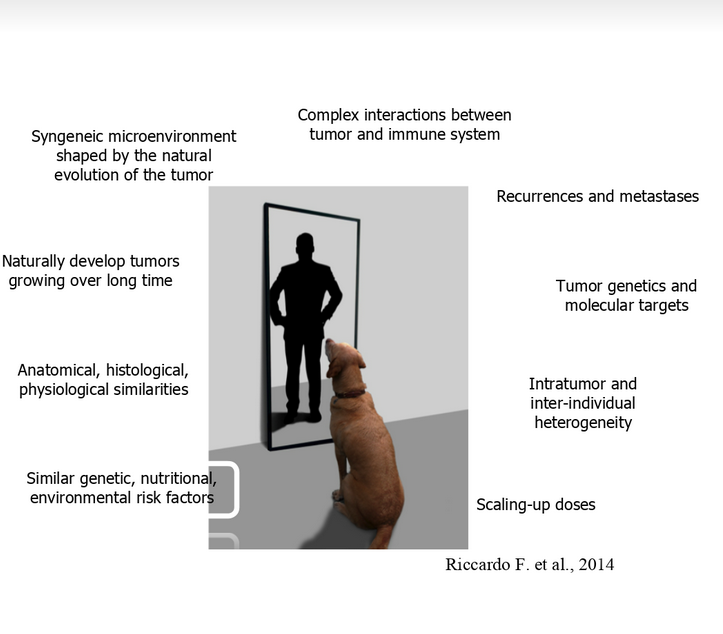
Modern oncology is based on the simple but at the same time innovative concept of “One health”, which recognizes the relevance of the similarities between diseases in humans and animals. In particular, tumors that develop spontaneously in dogs are extremely similar to human ones, both from a histological and molecular point of view, as well as for the clinical behaviour. Therefore, canine patients represent an excellent translational model for the development of new effective anti-tumor therapies that could simultaneously revolutionize the veterinary and human clinic. In this panorama of comparative oncology research rises out our project.