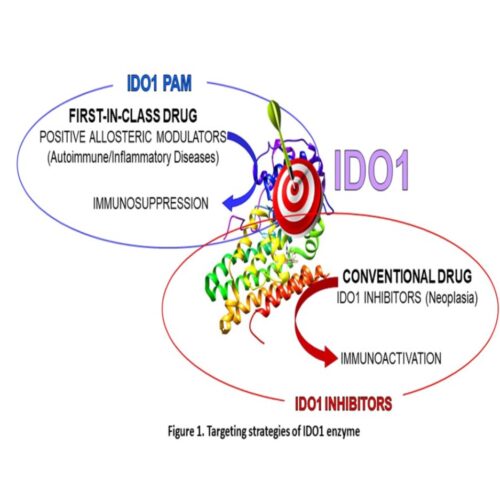
IBD is a highly prevalent disease in western society and a growing problem in emerging countries. It includes a group of disorders that cause chronic inflammation in the intestines such as Crohn’s disease (CD) and ulcerative colitis (UC). Although the underlying cause of IBD is multifactorial and remains largely unknown, it is attributed to an inappropriate immune response against environmental factors (e.g. dietary or microbial antigens) and genetic susceptibility. As such, so far, no long-lasting disease modifying therapies exist for IBD. Although multiple treatment strategies are available for IBD, all of them show indeed varying efficacy and safety. For example, aminosalicylates are well established for UC, but not for CD. Most critically, long term clinical remission of IBD is hardly reached. Patients have frequent relapses and remain sensitive to triggers. The thrust of our invention idea stems from the observations that (i) IDO1 is highly upregulated in the gut mucosa in response to IBD; (ii) several Trp metabolites have been involved in the pathophysiology of IBD; (iii) genetic polymorphisms of IDO1 have been associated with a severe clinical course of CD, suggesting lower IDO1 functions during the disease. These observations suggest that VIS329, acting as positive allosteric modulator of IDO1, may represent a disease modifying therapy for IBD.