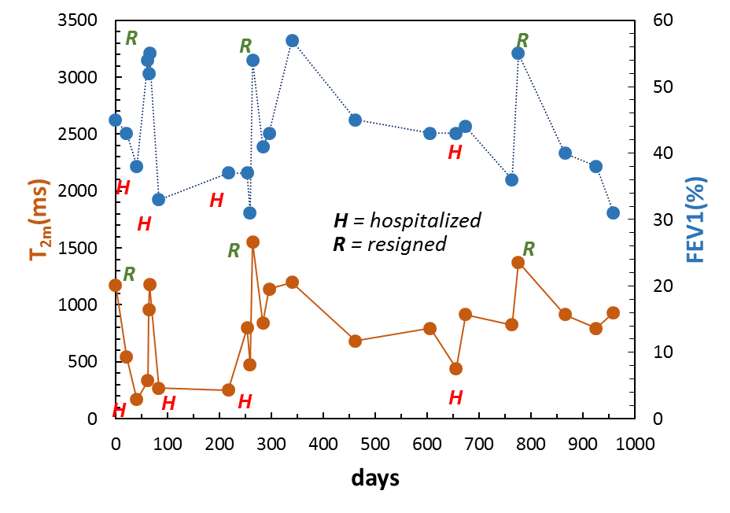
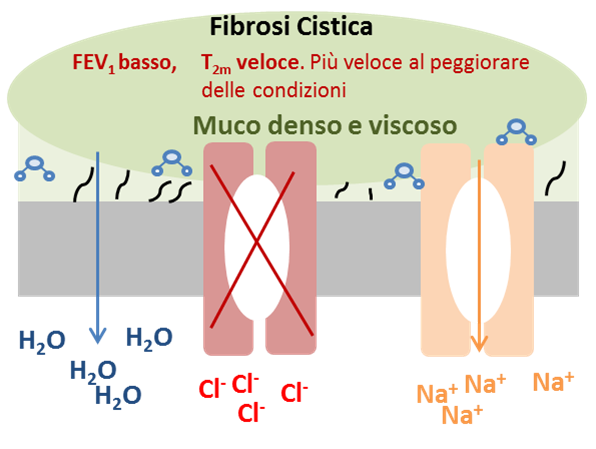
The invention consists in the use of low-field nuclear magnetic resonance (LF-NMR) for the monitoring of patients affected by cystic fibrosis (CF). The method makes it possible to indirectly evaluate the pulmonary function of patients with CF by analyzing a sample of sputum; the result is immediate and allows you to intervene immediately in the event of an infection, in order to prevent further lung damage.