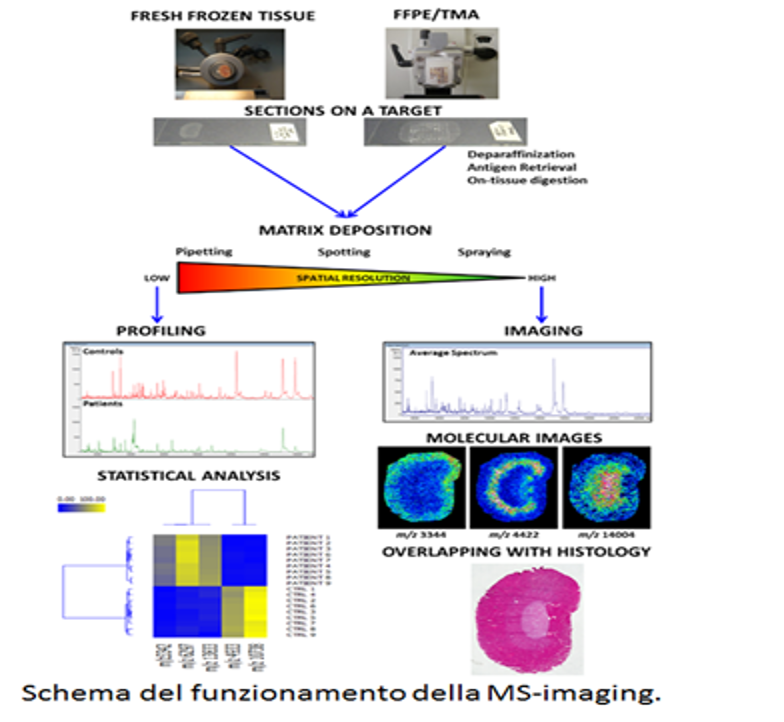
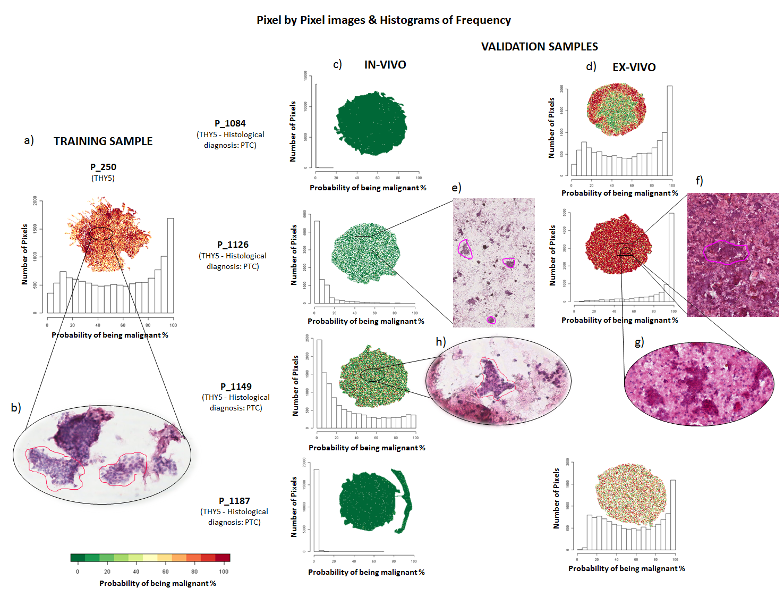
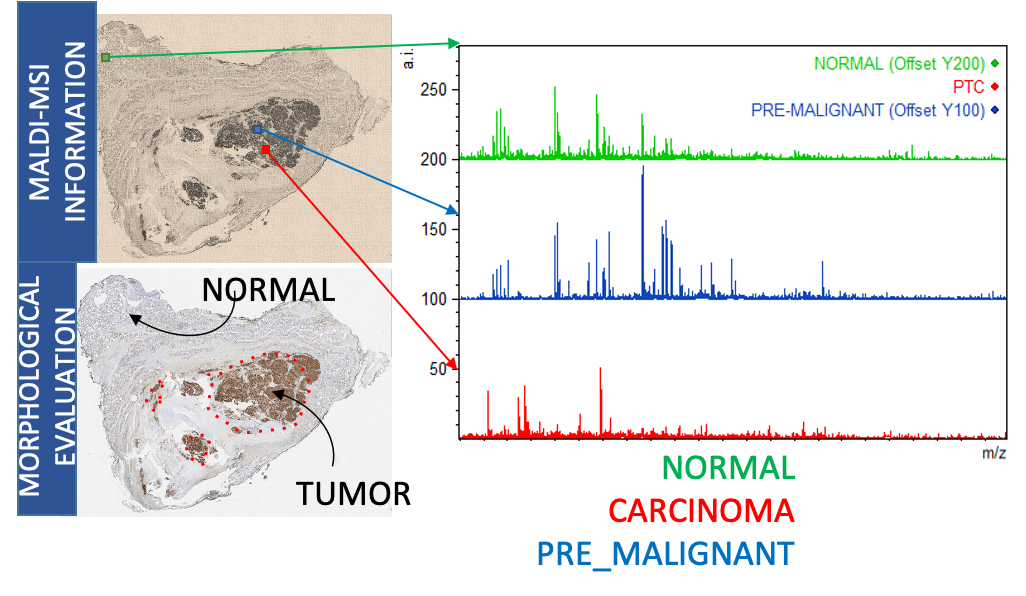
Since 2012 the UNIMIB Clinical Proteomics and Metabolomics Unit in collaboration with the Anatomia Patologica group has led not only to numerous publications in the most important sector journals and to the success of some competitive scholarships (FP7-PEOPLE-2013-ITN, AIRC MFAG 2016, PRIN 2017) also to the filing of a patent concerning a diagnostic method for the recognition of the “molecular signature” of malignant thyroid neoplasms (consisting of a combination of proteins expressed by tumor cells and not by benign nodules) based on proteomics through advanced mass spectrometry (MALDI-TOF; MS-Imaging). It allows a diagnosis of cancer on the same biopsy sample in which the conventional pathological analysis is indeterminate. Currently, after consolidating the methodological aspects, removing the interference due to the high amount of hemoglobin and stabilizing the biological material for at least 10-14 days in order to allow centralized analysis with sampling from different clinical centers, more than 200 samples. The statistical algorithm that discriminates between malignant and benign is being optimized with over 100 patients for the educational phase and another 100 patients for the verification phase. Within a few months we will have the definitive model for the subsequent clinical validation phase.