Our Technology and solutions
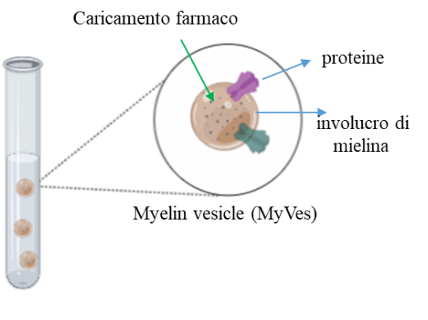
A protocol for the MyVes production was been developed. MyVes produced, were been characterized, from the chemical-physical point of view, in terms of size (˂100 nm), morphology (spheroidal), zeta potential (-37mV), stability ( > three months), and the presence of a phospholipid bilayer and proteins (20%).
MyVes vesicles have been partially characterized from a biological point of view, in fact they are cytocompatible following administration in neuronal cells and are able to interact with them. Finally, MyVes do not show a cytotoxic or activating effect on microglial cells.
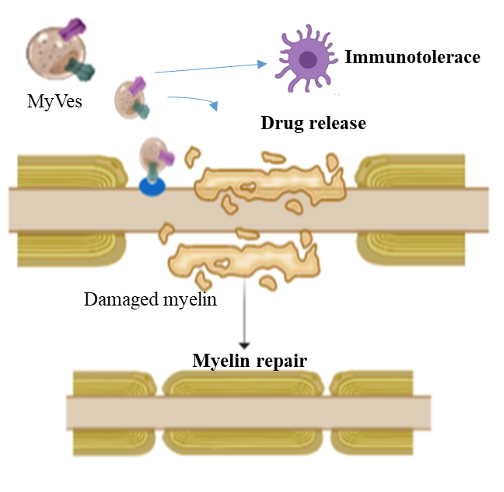
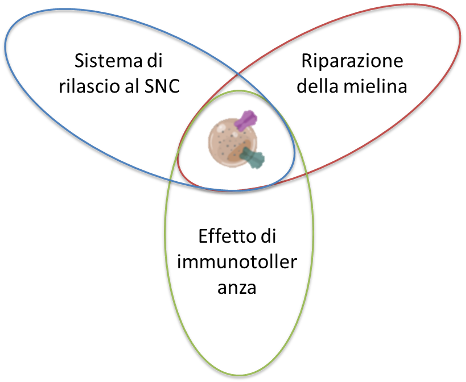
The peptides present in the vesicles may be able to direct the vesicle to the target site (interacting with the proteins exposed in the demyelinated neurons) and / or interact with the immune system and induce immunotolerant responses.
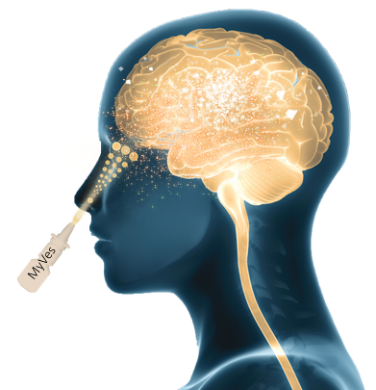
Thanks to their nature and chemical-physical and biological characteristics, we foresee that the Myves can counteract the demyelinating pathologies of the CNS, and we foresee a direct action on the CNS through the intranasal route (nasal spray), less invasive or intravenously crossing the blood brain barrier.