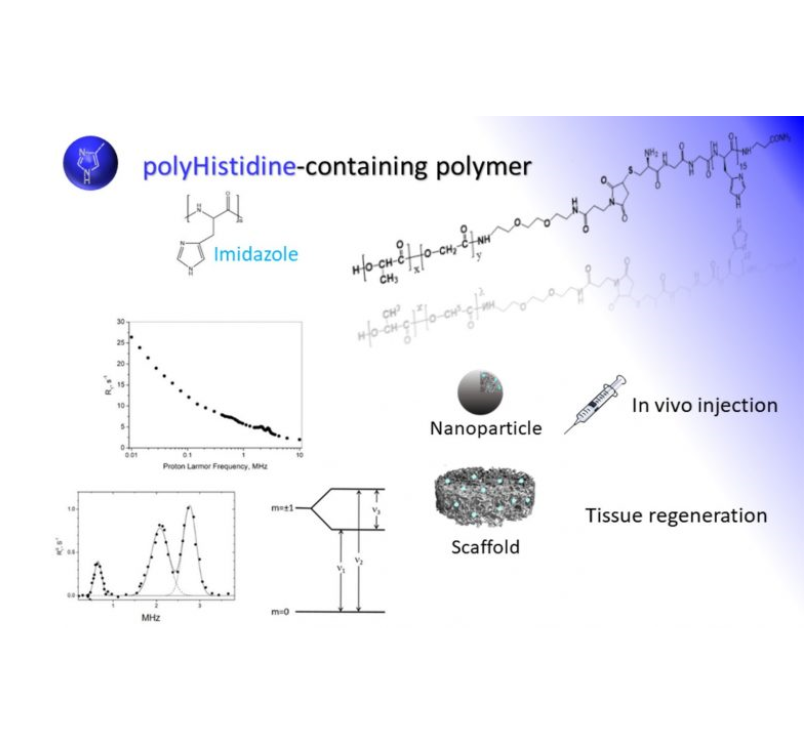
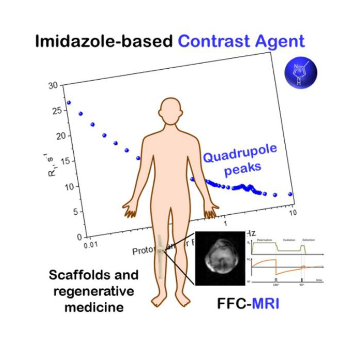
The compounds object of the invention are a new metal-free class of contrast agents for magnetic resonance, whose action is based on an innovative mechanism that do not use potentially toxic metals. The compounds work at fixed frequencies and they may be used for the design of nano- and micro- devices reporting on local pathological and physiological changes, in vitro and in vivo