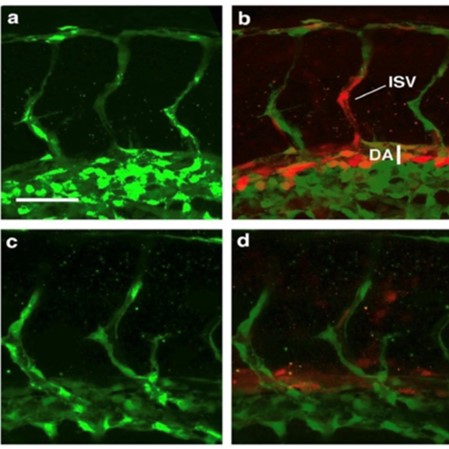
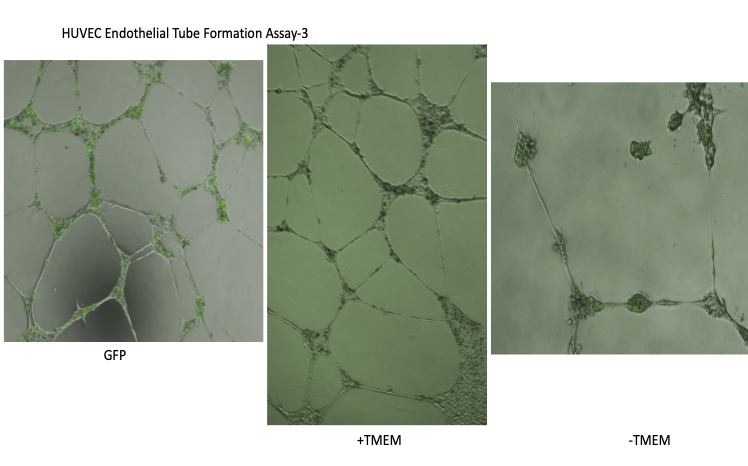
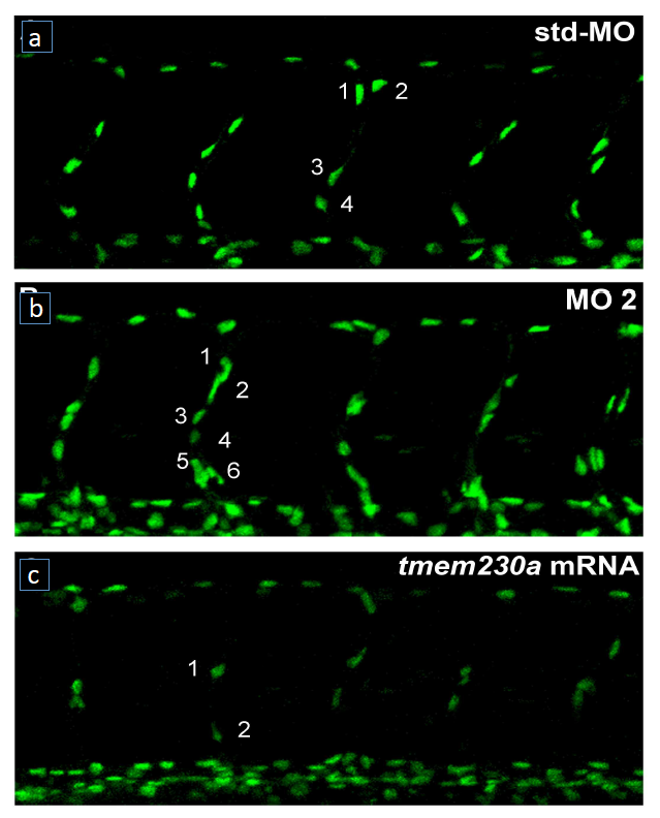
Tissue repair, organ regeneration, tumor formation and wound healing associated angiogenesis are regulated by the balance of signaling factors initiated by inflammation. Disruption or inhibition of the regulation of the balance of the diverse steps of normal angiogenesis such as sprouting, cell proliferation and 3D lumen structure formation results in aberrant and excess cell number, tissue remodeling and destruction and loss of normal tissue function.
The molecular components of normal and chronic wound healing are not completely characterized, yielding mostly unsatisfactory or ineffective treatments for acute or chronic tissue damage such as those associated in spinal cord injury, chronic inflammation induced by pathogenic infection or cytotoxic factors, autoimmunity disorders such as rheumatoid arthritis and neurodegenerative disorders.
Identification of novel molecular components of normal and chronic wound healing will contribute new meaningful markers for diagnosis and prognosis and therapeutic targets for acute and chronic injury, and in addition tumor and cancer treatment.