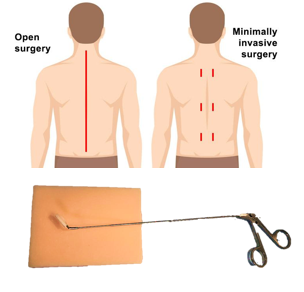
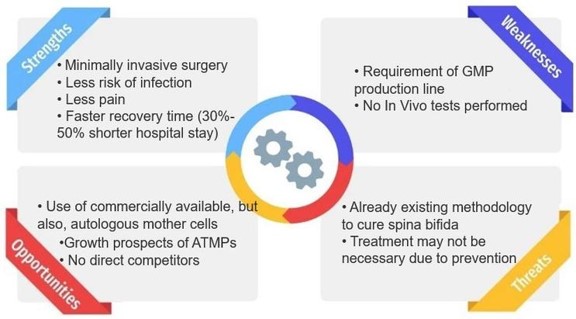
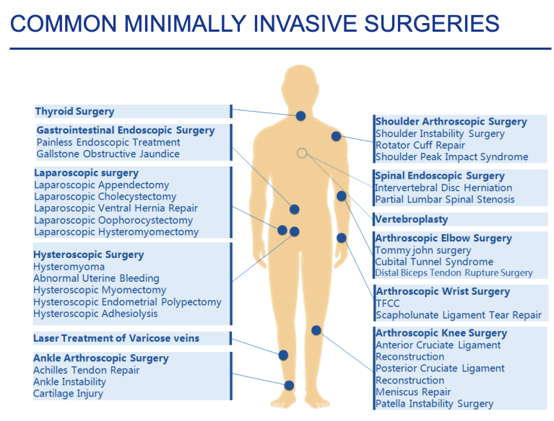
The present invention consists of a method for the production of a shape memory engineered tissue (SMET) through the use of electrospun polymeric matrix capable of transiently modifying its shape, following the application of an external stimulus such as temperature, and at the same time capable of supporting the delivery of cells and/or drugs. SMET can be used as as mini-invasive device for tissue regeneration.