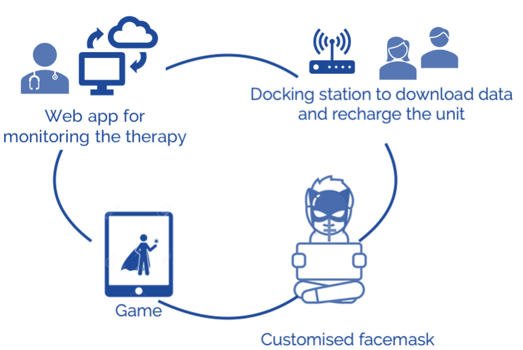
Albeit effective, currently available facemasks on the market have several critical issues resulting in the child’s lack of cooperation in wearing the therapeutic appliance for the prescribed time (12 or 24 hours a day, for 9 to 12 months). Available in only two standard sizes, these masks are poorly ergonomic, being heavy and often ill-fitted to the forehead and chin. The forehead and chin supports often causes irritation and decubitus. Consequently, the perceived discomfort, bulkiness, and instability of the mask, in addition to poor aesthetics, represent additional sources of shame and embarrassment on the part of young patients.
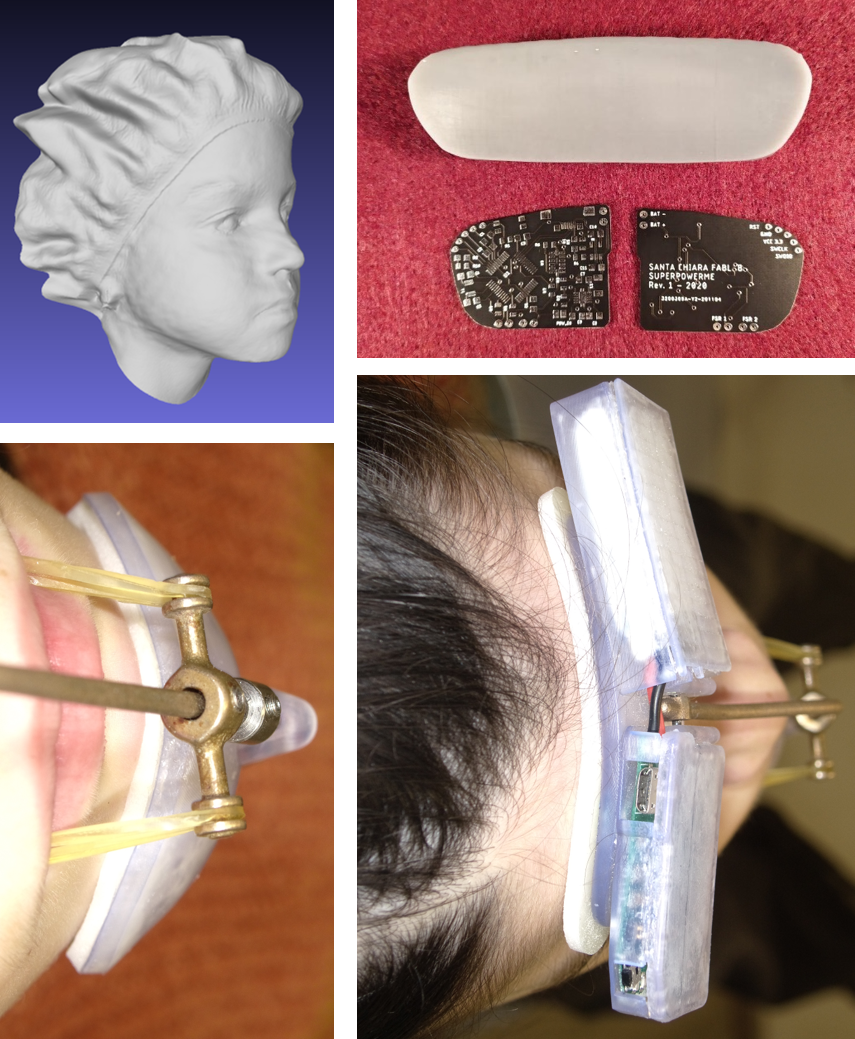
Previous customization strategies include recording an alginate impression of a patient’s face, and the derived plaster model is used to fit the facemask components. Nevertheless, when in direct contact with the skin, alginate can be a source of discomfort for the patient. Alternatively, a PVC-based putty material applied to the inside of standard supports improved the fit of the pieced by taking the natural contours of the face, though not further facilitating treatment compliance.