Acute Kidney Injury (AKI) is a syndrome attacking kidneys causing a rapid decline in their functional capabilities. In most cases AKI occurs in conjunction with other diseases, typically related to cardiovascular and respiratory systems. For this reason, patients with the highest risk of incurring in AKI are older people, diabetic people, or people affected by hypertension and those having heart failure.
In a general context of critical medical status of the patient, the lack of the function of purification and regulation carried out by the kidneys worsen the clinical condition turning out to be a fatal aggravating, contributing to the death of the hospitalized in 13% of cases and causing a 10 fold increase in mortality rate.
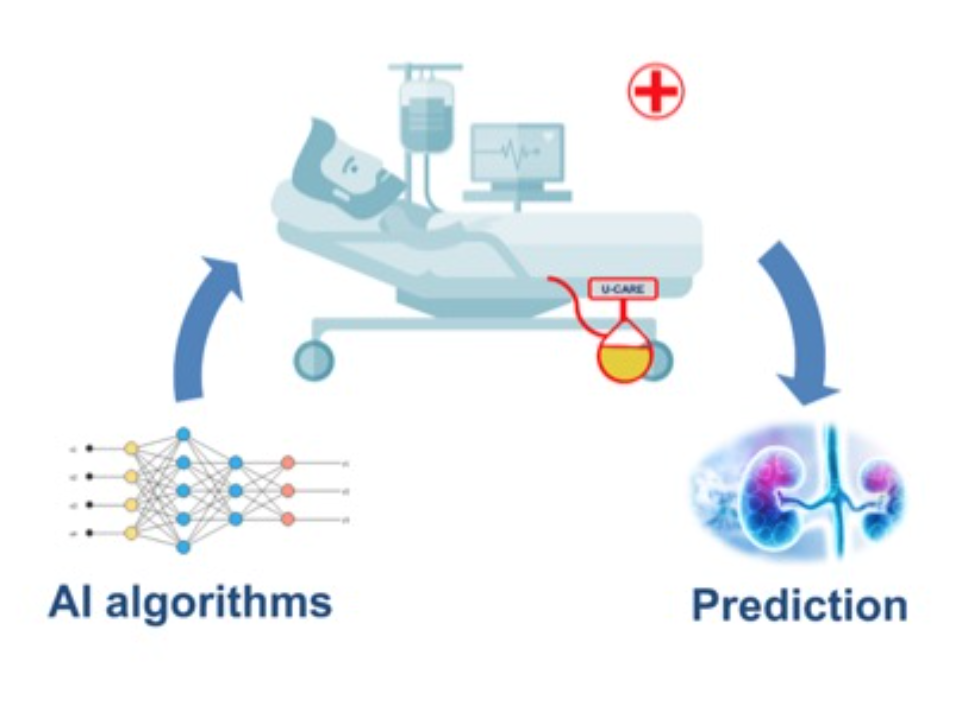
The main problem related to AKI is the difficulty in having a timely diagnosis of the syndrome. Indeed,
the gradual loss of renal function, which leads to an AKI episode, is not clinically observable by monitoring physiological parameters or by looking at clinical examinations results. Therefore, the physician is not able to conduct specific therapies to prevent AKI, since there isn’t information about renal function and the risk of getting AKI.