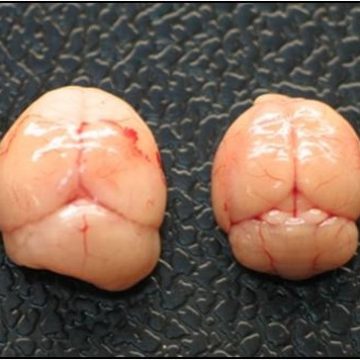
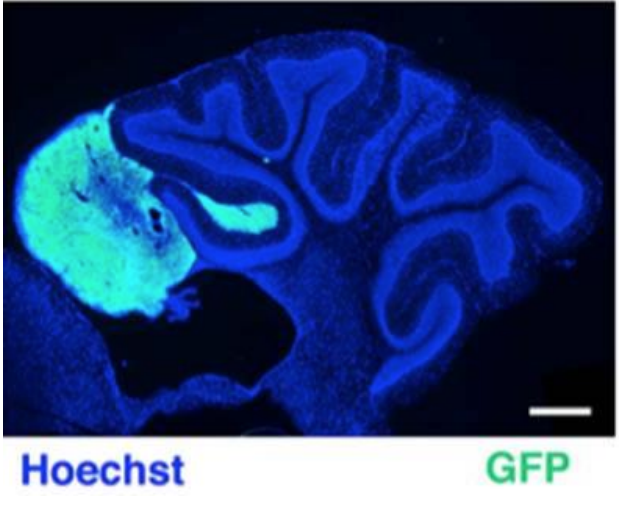
Medulloblastoma (MB), tumor of the cerebellum, is a leading cause of cancer related mortality in childhood. In a mouse model of spontaneous MB, correlation between a defect of the migration of cerebellar precursor cells (GCPs) and an increased frequency of MB has been demonstrated. The chemokine Cxcl3, responsible for the inward migration of GCPs, can suppress the development of the MB lesions.