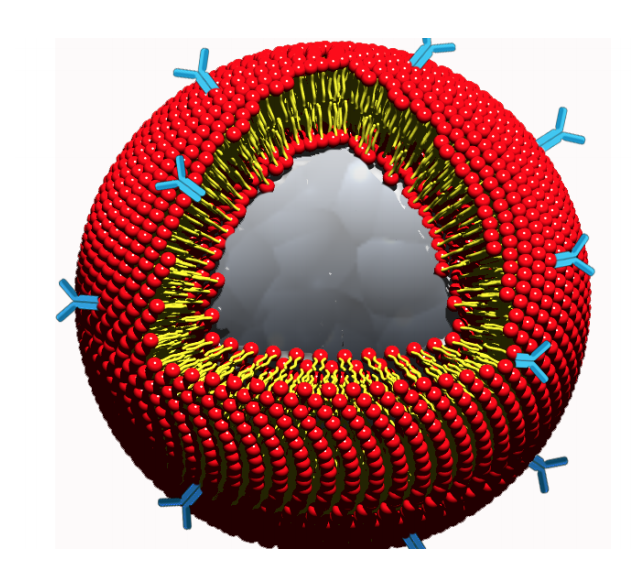
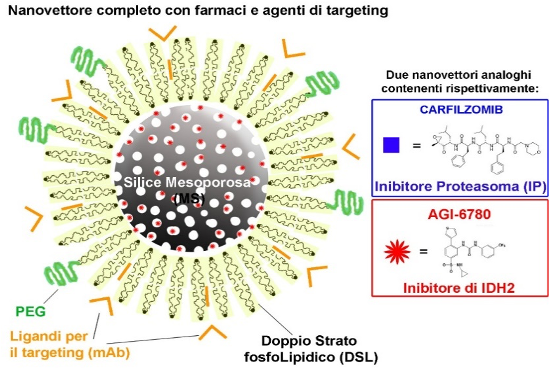
FOCUS ON MULTIPLE MIELOMA: Among the present solutions, «conventinal» therapies are in place:
Chemiotherapy: Oral or endovenous administration of drugs. For multiple mieloma, mono-therapy or combination therapies with PROTEASOME INHIBITORS (PI: Bortezomib, Carfilzomib, Ixazomib ) + Immunomodulatory imide drugs (IMiDs: Lenalidomide+bortezomib). Disadvantages are disease progression, frequent relapses, drug resistance development.
Autologous Stem Cell transplantation: Treatment able to originate a new bone marrow, reconstituting the hematopoietic system of the patient, which is strongly compromised by this disease. This therapy is only partially efficaceous, is combined with chemotherapy in high doses, and is not applicable to other tumors, i.e. solid ones.
Car-T (Chimeric Antigen Receptor) cell therapy: It acts on the immune system of the patient enabling the recognition and fight of the tumor cells. It is a new experimental therapy, personalized for the patient, but it is characterized by very high costs and requires highly specialized laboratories and biotech companies.