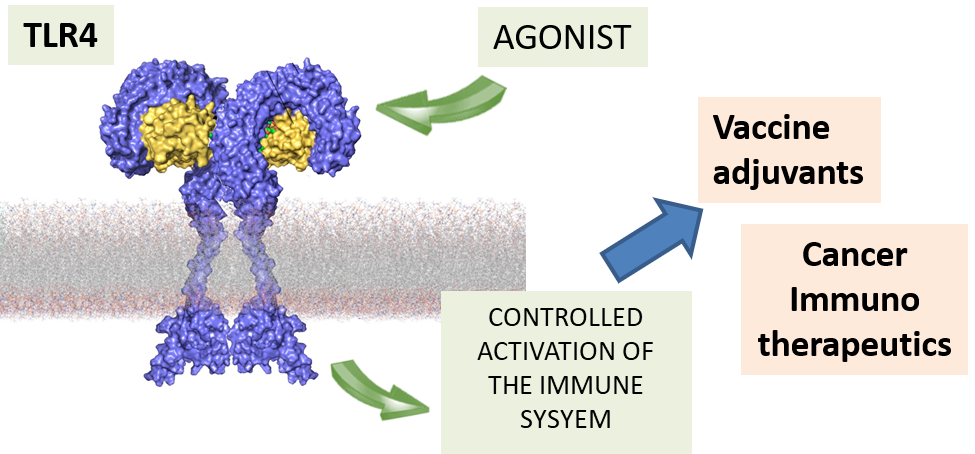
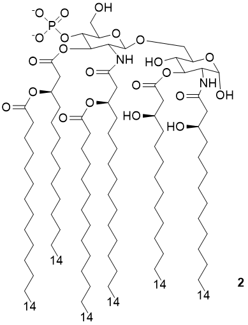
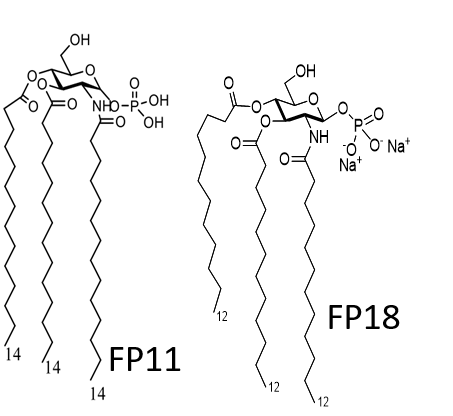
The invention relates to the synthesis and the biological/pharmacological activity of new molecules active in stimulating the human receptor TLR4. This receptor is one of the key players in activating inflammation and immune response to bacterial pathogens in humans. The capacity of the synthetic molecules described in the patent (called FP molecules) to stimulate TLR4 and their lack of toxicity, made them interesting lead compounds as immunostimulants for vaccine adjuvants development.
A collaboration is running with Croda/Avanti for the development of nanoformulated FP molecules as vaccine adjuvants to reach the veterinary market and eventually to access to clinical phases.