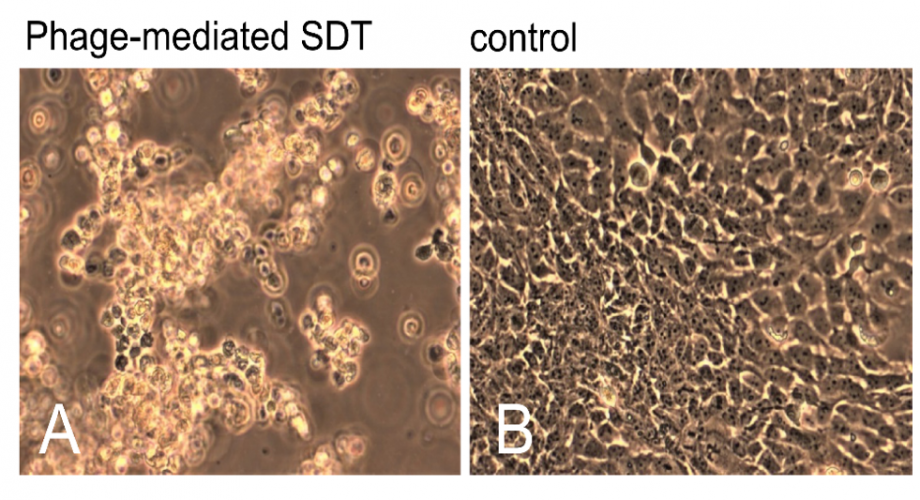
Sonodynamic therapy (SDT) is an innovative therapeutic approach involving a combination of ultrasound and specialized chemical agents known as sonosensitizers. Activation of the sensitizer by ultrasound generates reactive oxygen species (ROS) responsible for cytotoxicity. Because ultrasound can penetrate deeper into tissues than light irradiation, it allows treatment even in deep regions of the body. As an example, ultrasound can be focused on a region of a tumor to activate a sonosensitizer, thus offering the possibility of targeting solid tumors.
One of the limitations strongly felt with SDT, however, is related to the high ultrasound powers that must be employed to achieve enough sensitizer activation to be effective. Conversely, excess ultrasound power also damages healthy tissue. In addition, the sensitizers must be selectively targeted to target cells so as not to damage surrounding healthy cells. A solution (vector) that would allow low ultrasound powers to be combined with specific targeting of a sufficient number of sensitizers would therefore have a disruptive impact in the field of SDT, with a truly wide court of applications and patients benefiting from the advantages offered by the solution.