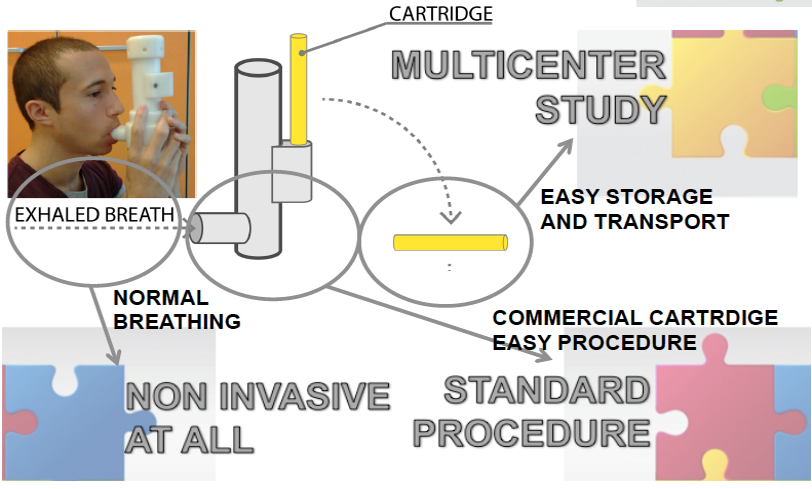
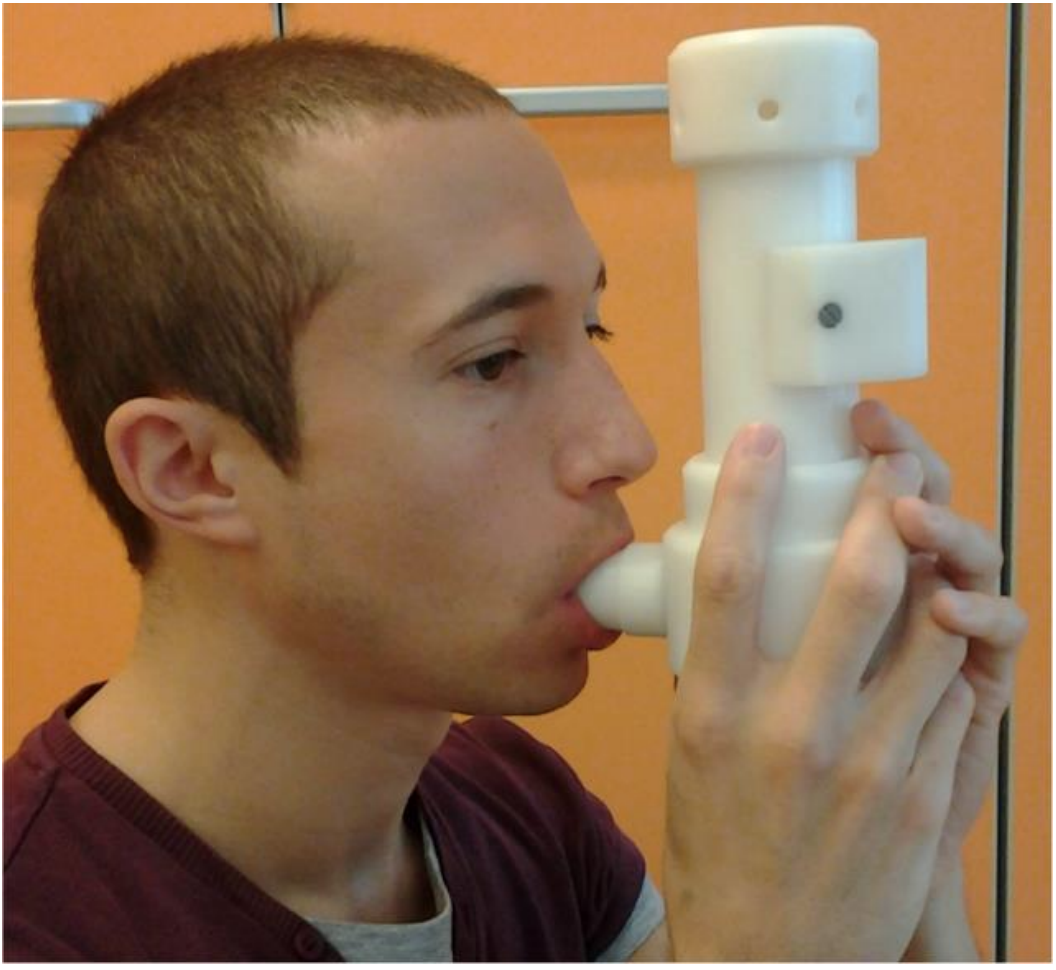
Device for non invasive collection of exhaled breath. The individual has to breath normally into the Pneumopipe for three minutes. After this 3 minutes the exhaled breath has been collected into a cartridge which can be stored, delivered or measured by thermally desorbing its content into an instrument for gas and vapour analysis.
Pneumopipe is a prototype currently sitting between TRL4 and TRL5, which has been validated in relevant environments. So far a library of more than 2000 breath fingerprints have been built by performing measurement campaigns on different diseases in different hospitals, including the assessment of comorbidities and confounding factors.